Fusion QbD®
Fusion Inhaler Testing
Software for Oral and Nasally Inhaled Drug Product R&D and QC
Links to More Information about Fusion Inhaler Testing!
Product Brochure (PDF | Online) • Part 11 Compliance
Fusion Inhaler Testing (FIT) is the only OINDP software which supports the additional Interpolation-Regression approach to calculating MMAD and GSD specified in ISO 27427. (Page 25)
USP General Chapter <1604> contains the two important elements listed below.
- Pre-separator stage calculations for NGI Apparatus with pre-separator — flow rates from 30–100 L/min. (Table 2B, Page 10)
- Calculating MMAD when the raw data distribution is not log-normal. (Page 11)
The change in Item 1 above impacts several important calculations based on particle-size data obtained from ACI and NGI impactors for flow rates from 30–100 L/min. The new guidance 1) provides a cutoff diameter for the pre-separator stage, and 2) excludes Stage 1 from the stages used in the calculations when the apparatus configuration does not include a pre-separator. The USP committee is currently discussing this topic, and therefore recommends not making any changes to current practice until they provide an update in 2025 — either in a revised chapter or at a USP workshop. S-Matrix will immediately incorporate any final guidance updates into our Fusion Inhaler Testing Software Platform (FIT). The next release of FIT will support the calculation approach described in Item 2 above.
S-Matrix – Software Solution Partner of:


Works with the following Chromatography Data Software:
- ChemStation and OpenLab 1.x – Agilent LC Systems
- Chromeleon™ 7.2 (SR5 or later) – Thermo Scientific and Agilent LC Systems
- Empower™ 3 – Waters® and Agilent LC Systems
INHALER TESTING (OINDP R&D)
Additional Toolset Option for the Fusion Process Development Module (FPD)
With this toolset you can automatically:
CLOSE- Create Testing Plans for respiratory drug and device testing results data such as are obtained from ACI and NGI cascade impactor testing.
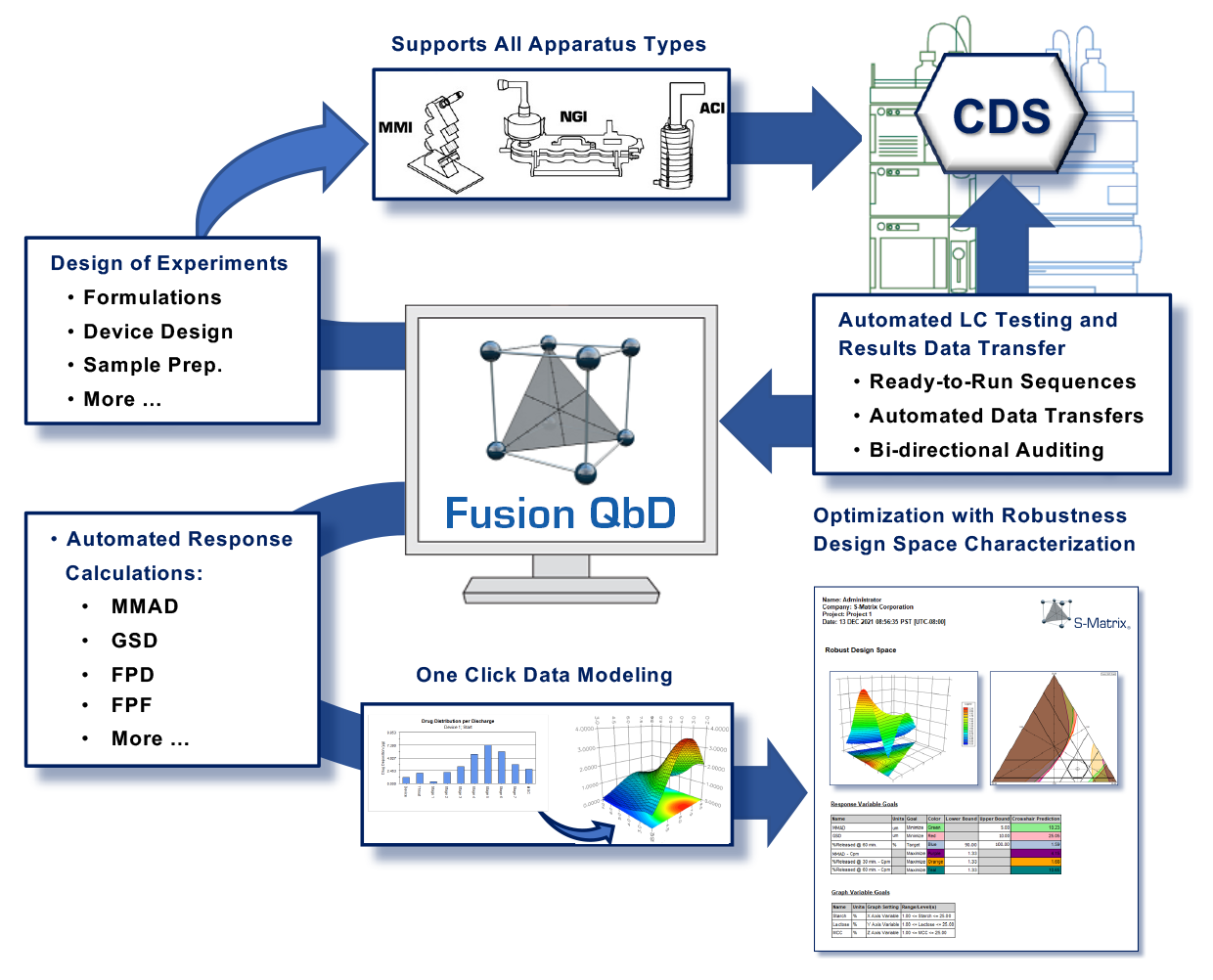
The Inhaler Testing Toolset can automatically create Testing Plans for:- USP Apparatus 1-6 and Ph.Eur. 2.9.18 Apparatus C, D, E.
- any combination of device by stage of dose delivery life
- Exchange LC testing plans and chromatogram results between Fusion QbD and your Chromatography Data Software (CDS) with full Part 11 compliance support, data integrity, and bi-directional auditing.
- Export the Testing Plans to the CDS as ready to run sequences which include your standards injection strategy
- Import all chromatogram results automatically from the CDS with full data tracking and audit logging
- Compute all your critical particle size distribution results from the chromatogram results:
- compute apparatus stage and group averages, Material Balance, Mass Balance, Metered Dose, Emitted Dose, Actuator/Device Retention, Fine Particle Dose (FPD), Fine Particle Fraction (FPF), Mass Median Aerodynamic Diameter (MMAD), and Geometric Standard Deviation (GSD)
- map all computed responses to the experimental design for automated data analysis
Fusion Inhaler Testing (FIT)
Fusion Inhaler Testing (FIT) creates Device Sampling Designs for metered-dose inhalers (MDIs) and dry powder inhalers (DPIs). It supports USP Apparatuses 1–6, Ph.Eur. Apparatuses C, D, and E, and Dose Unit Sampling Apparatuses (DUSAs). FIT also creates coordinated HPLC Testing Designs that are directly exportable to your Chromatography Data Software (CDS) — connectivity includes Agilent ChemStation, Dionex Chromeleon, and Waters Empower 2 and 3.
Key Benefits
- Automates all QC Testing on HPLC/UHPLC
- Minimizes Manual Data Transcription Work and Error Checking
- Supports 21 CFR p11 compliance
- Saves 2–3 Days per Week per Analyst!
Example Applications
- Drug Formulations
- Dosage Delivery Devices
- Airborne Pollutants
FIT Export operation automatically builds the sequence (or sample set) in the CDS, adds the appropriate standards injections, and attaches the LC Assay Method so that the sequence is ready to run on the LC in full walk-away mode.
FIT automatically imports all chromatographic results from the CDS and generates all results and graphs typically required for inhaler testing. Key features include regulatory-compliant reporting, output formats such as *.PDF and *.DOC, a full 21 CFR 11 compliance toolset, and a workflow management system.
FIT enables users to manage all of their apparatuses and product testing configurations:
- Input apparatus-specific Mensuration data and associated expiration dates. The program can automatically calculate and store the effective cutoff diameter data for an apparatus from the Mensuration data, and communicate these data to each product testing project.
- Create product-specific Product Testing Configurations to which multiple apparatuses can be assigned.
- Easily map individual testing apparatuses to their associated test data. The program then automatically retrieves and uses the apparatus-specific cutoff diameter values during data analysis.
- Create analysis templates which automatically generate and report results in FDA/USP or EU formats, as well as General, which enables selection of any or all of the possible analysis calculation approaches.
- FIT is also the only inhaler testing software product that provides full support for 21 CFR Part 11 Compliance!
FIT was developed in cooperation with several international pharmaceutical and medical device companies to address all USP and Ph.Eur analysis and reporting requirements and be consistent with current cGXP best practices.
Pharma Customer Benchmarking
To benchmark time savings using FIT versus current practice, an international pharmaceutical company repeated a standard set of testing, analysis, and reporting for an inhaled drug product testing protocol. Their estimated time savings using FIT was 40%. In addition, the automated data exchanges with the CDS virtually eliminated the possibility of transcription errors.
