S-Matrix Analytical Development Labs
Overview
Major Pharmaceutical companies worldwide use our Fusion QbD® Software Platform (Fusion QbD) every day to successfully develop truly robust and transferrable methods, including enterprise deployments at more than half of the world’s largest pharmaceutical companies. In addition, regulatory agencies use Fusion QbD to modernize methods and to challenge robustness claims in Pharma company submittals!
Our proven capabilities are now available to you as a service!
S-Matrix Analytical Development Labs (ADL) takes a unique approach to method development:
- All work is done using Fusion QBD, which implements a Quality by Design (QbD) approach 100% aligned with regulatory guidances and expectations, and enables us to rapidly develop truly robust and transferrable methods.
USP <621>, <1210>, <1220>, ICH: Q2(R2), Q8 – Q12, Q14 - All work can be done using the Chromatography Data Software (CDS) you use in your labs:

Key Differentiators of our Service
- Define Your Analytical Target Profile (ATP)
We work with you to define quantitative statements of your required method performance. The ATP is used to assess project progress, and is the basis of your verification and acceptance of the final delivered method.
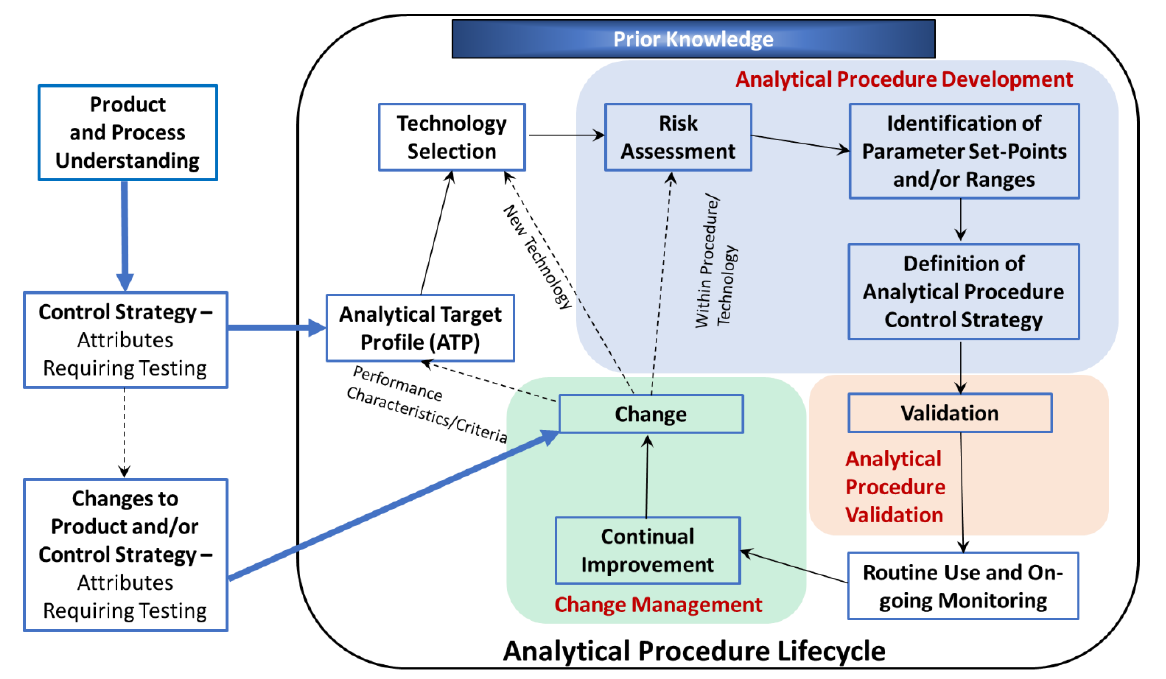
Analytical Target Profile (ATP)
An ATP consists of a description of the intended purpose of the analytical procedure, appropriate details on the product attributes to be measured and relevant performance characteristics with associated performance criteria. The ATP includes measurement requirements for one or more quality attributes.
- Integrate Quantitative Metrics of Method Robustness
We integrate robustness modeling for all key performance characteristics — as specified in the ATP — into method optimization to assure successful validation and transfer.
- Perform a Full Suite of Pre-validation Experiments
We qualify the method’s performance according to established FDA and ICH guidances to verify the ultimate validatability of the method. These experiments include:
- Analytical Capability*
- Specificity
- Filter Validation
- Sample Solution Stability
- Accuracy*
- Range (Linearity)
- Repeatability*
- Accuracy/Linearity/Repeatability*
- ICH Q2(R2) – can be done as a single combined experiment
- LOQ, LOD
- Intermediate Precision & Reproducibility (USP Ruggedness)
- Robustness – done the right way!
*Method Transfer Studies — Support Includes USP ⟨1210⟩
Tolerance &
Prediction Intervals
- Fully Document and Report all Work, Data, and Results

Physical Location
S-Matrix ADL
12368 Hancock Street
Carmel, IN 46032
USA
Phone: 707-441-0404
Email: sales@smatrix.com
Mailing Address
S-Matrix Corporation
1594 Myrtle Avenue
Eureka, CA 95501
USA
Phone: 707-441-0404
Email: sales@smatrix.com
