Fusion QbD®
Quality by Design Software Platform
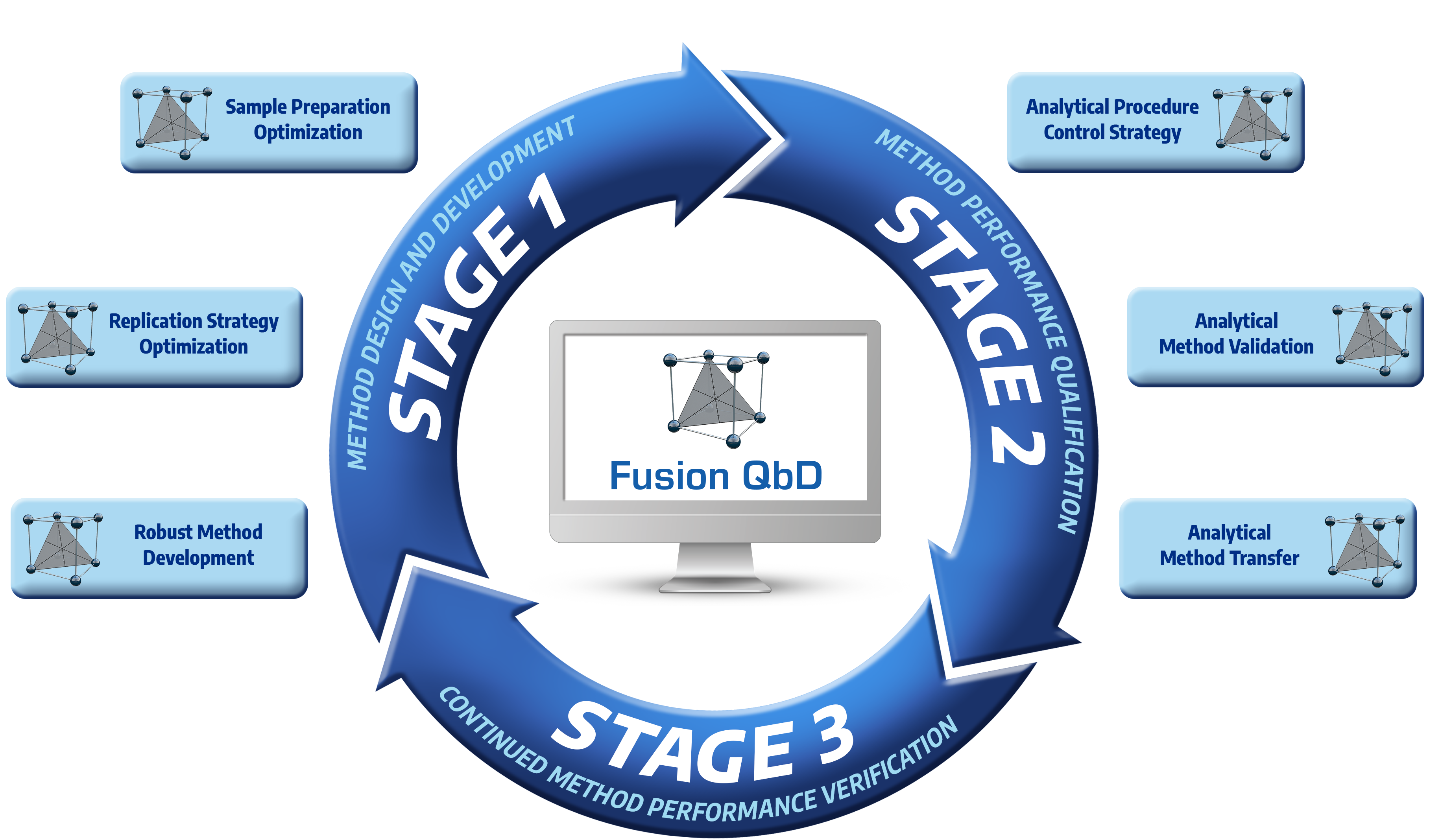
Chromatography Modeling and Optimization Software for the
Development, Validation, and Transfer of Analytical Methods.
- LC, LC-MS, SFC
- Sample Preparation
- GC, CE, Dissolution, and More...
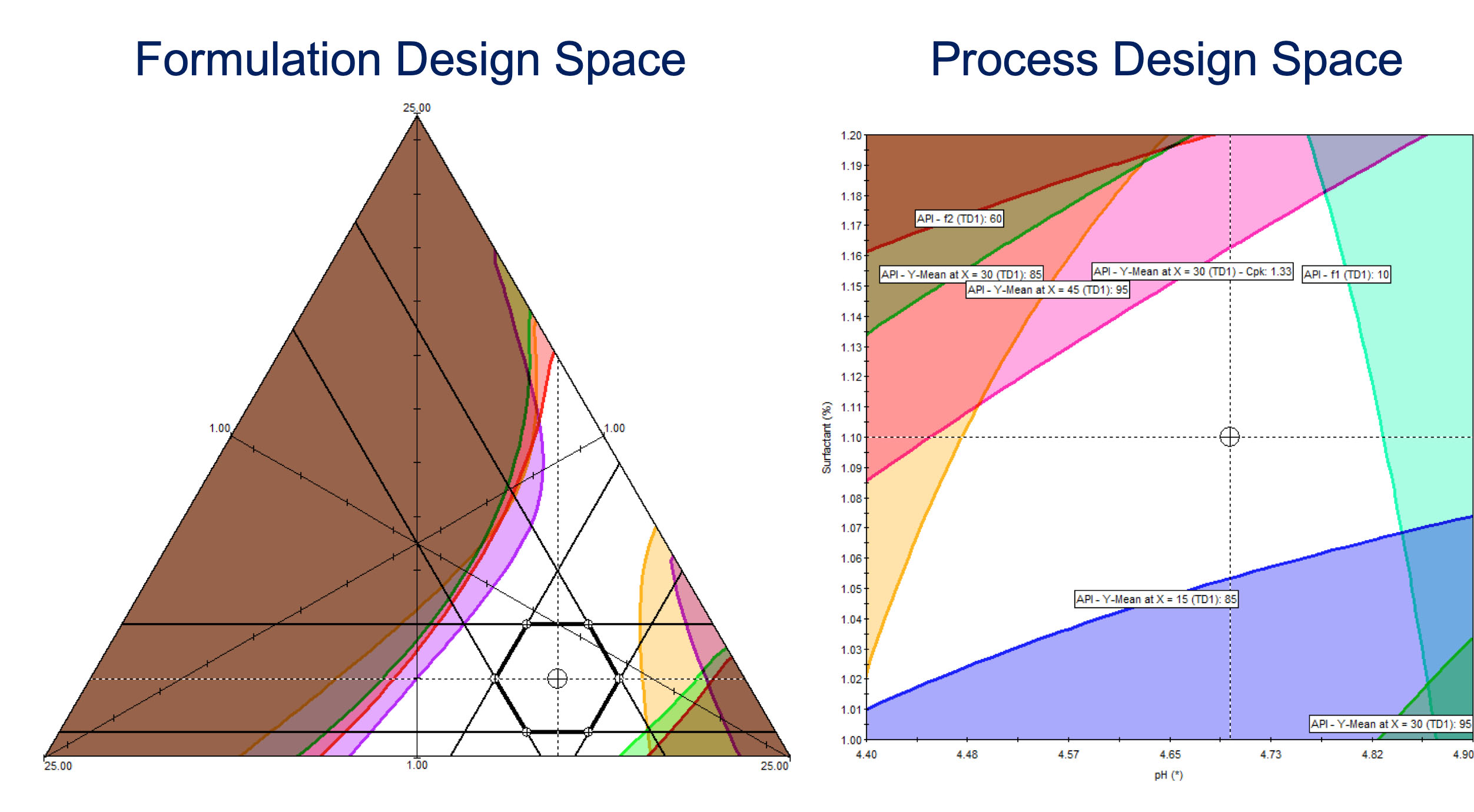
Development and Optimization of
any Formulation or Process.
Pharmaceutical Applications Include
Synthesis, Oral and Inhaled Dose Formulations, Granulation, Tableting & Coating, and More...
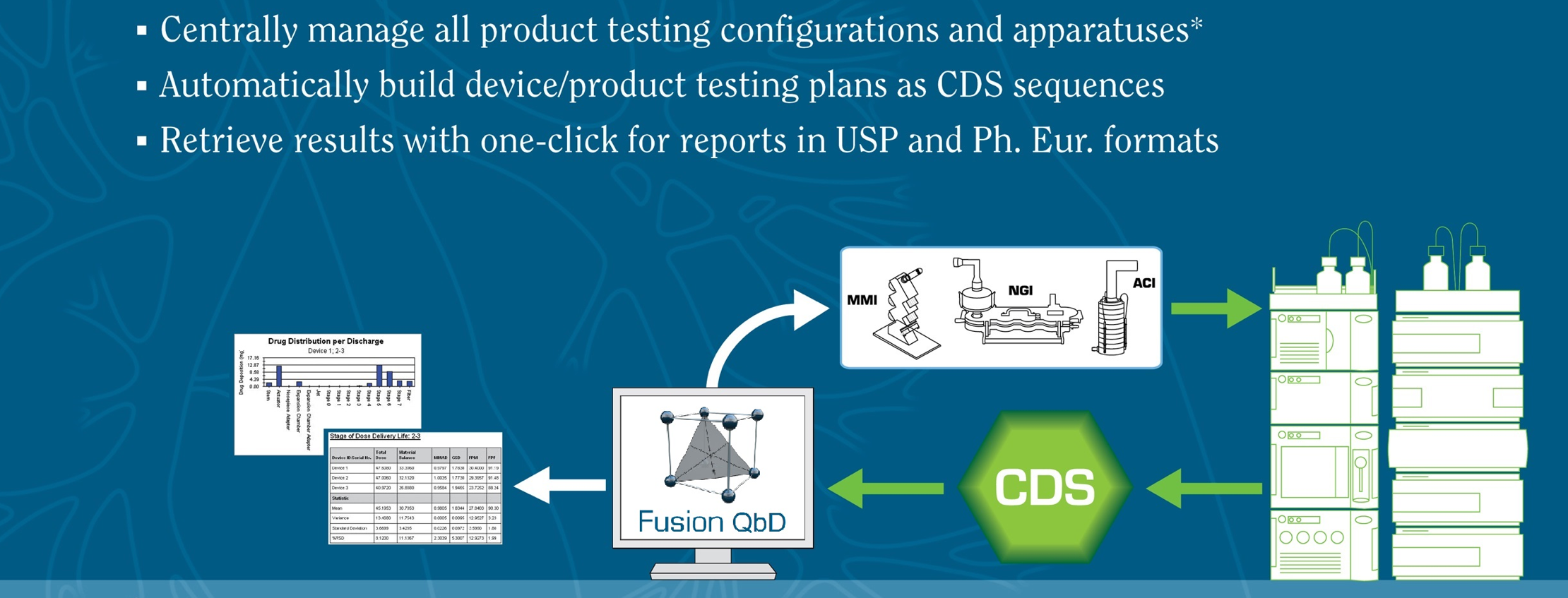
Development and Optimization of
Inhaled Dose Formulations & Device Designs.
QC Testing & Reporting with Full LC Automation and Full Regulatory Compliance Support.
Supports All Apparatuses, Manages Mensuration Data, Workflow Templating, and More...

Major Pharmaceutical companies worldwide use Fusion QbD every day to develop truly robust and transferrable methods.
Regulatory agencies use Fusion QbD to modernize methods and to challenge robustness claims in Pharma company submittals!
These proven capabilities are now available to you as a QbD Method Development Service!
Visit our Part 11 Compliance page for more information on Fusion QbD regulatory compliance and software validation by international pharmaceutical company customers and instrument vendor partners.
Fusion QbD is Citrix Ready™ Verified. Click here to read about Fusion QbD compatibility with Citrix.
Fusion QbD can be fully qualified in any supported deployment environment. Click here to read about Fusion QbD software qualification products and services.




