S-Matrix – Software Solution Partner of:






S-Matrix is the World Leader in QbD Experimentation Software for the development, validation, and transfer of LC, LC-MS, and SFC methods.
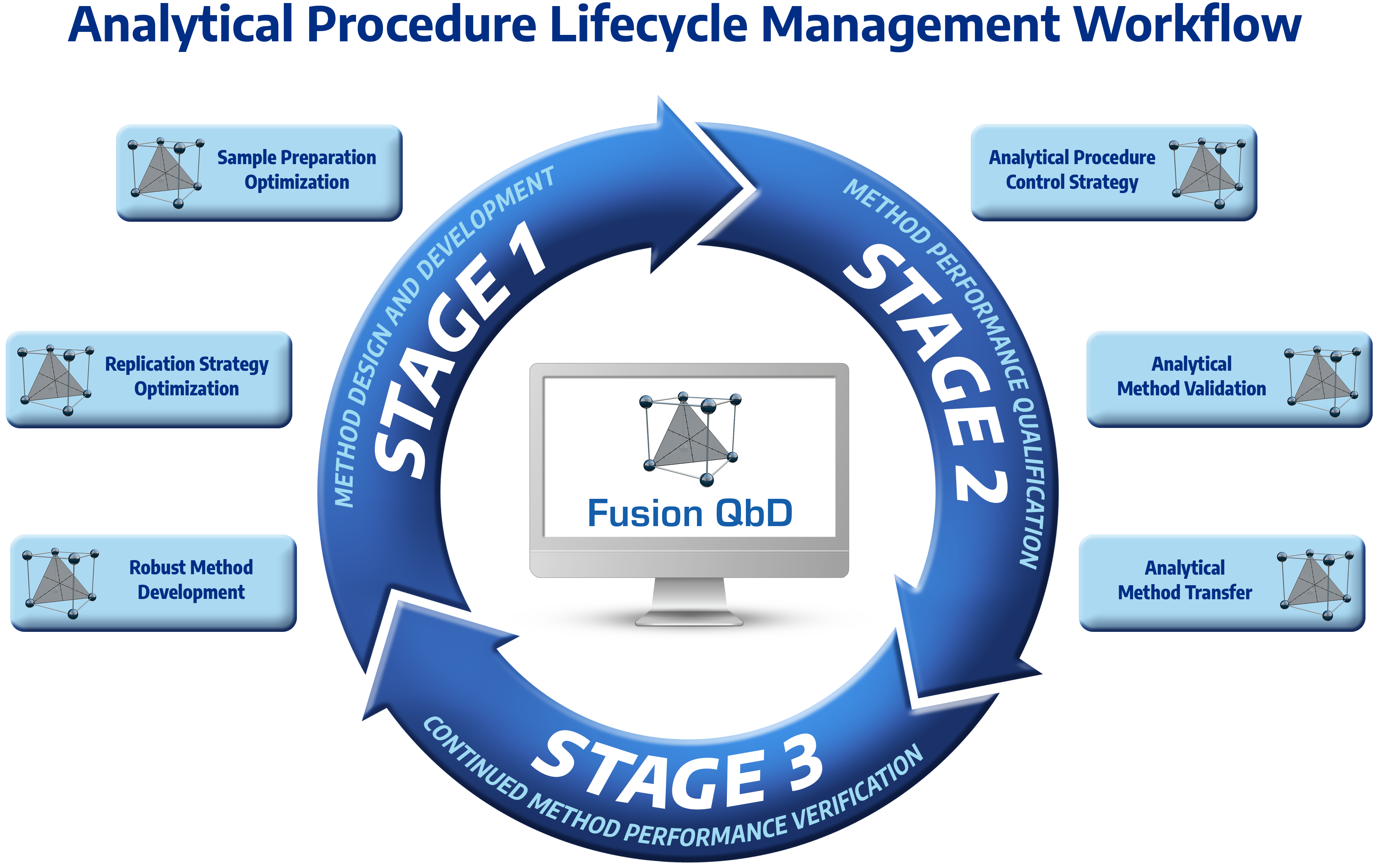
Fusion QbD Supports Full Compliance and Data Integrity in Perfect Alignment with all Regulatory APLM and AQbD Guidances
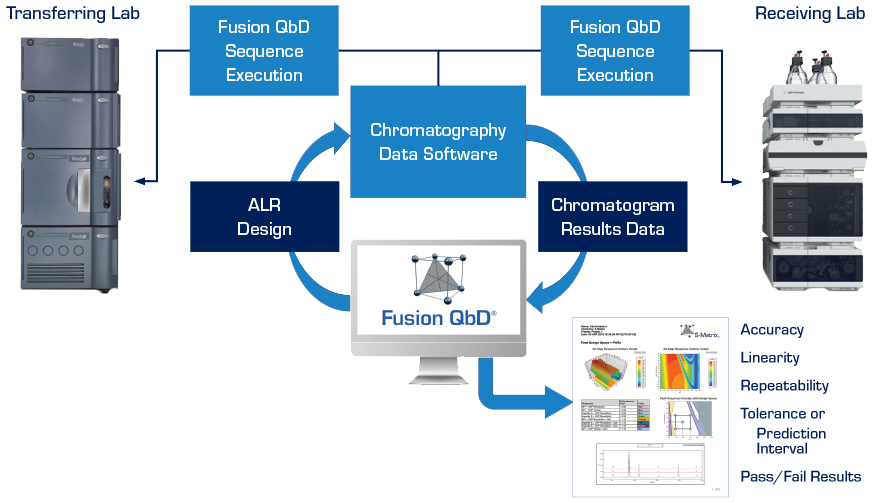
The only software platform that is 100% aligned with the Analytical Procedure Lifecycle Management (APLM) model and supports full closed-system regulatory compliance, including bi-directional auditing with the CDS, to meet regulatory expectations of required data integrity.
Fusion QbD Perfectly Integrates DOE, Chromatography Modeling, and Experiment Automation
The only software platform with a complete and perfect integration of Design of Experiments methodology (DOE), Chromatography Modeling, and Experiment Automation on Agilent, Thermo, and Waters LC systems for fully automated walkaway experimenting.
Fusion QbD Supports all Phases of LC Method Development
The only software platform that supports all phases of your LC method development work, including Sample Preparation, Initial Sample Workup, Chemistry System Screening (also called Method Scouting), and Robust Method Optimization using in-silico Monte Carlo robustness modeling of all critical method performance characteristics to establish the Method Operable Design Region (MODR) — the analytical method’s robust design space.
Fusion QbD Fully supports all Chromatographic Separation Modes for Both Small and Large Molecules
The only software platform that supports all separation modes for both small and large molecules, including RPC, NPC, IEX, SEC, HILIC, HIC, Chiral, and SFC. Fusion QbD is used successfully for Peptide Mapping, mAbs, and also for development of Non-LC methods such as CE, GC, and Dissolution.
Fusion QbD Includes Fully Automated UV and MS Spectra Based Peak Tracking
The only software platform that supports fully automated peak tracking using traditional peak data, UV spectra data, and MS spectra data from the Waters QDa, SQD, and SQD2 Mass Detectors – support for other MS systems will be coming in 2022.
Turn Your LC System into a Method Development Powerhouse
Click here to see a history of Fusion QbD automation.
Rapid Chemistry Screening – Column, pH, Gradient, Mobile Phase
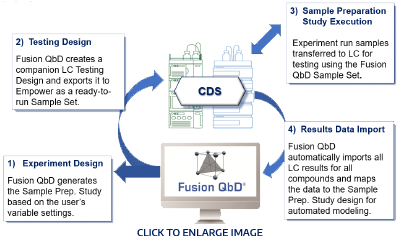
Fusion QbD® brings a completely new quantitative approach to automated LC column and solvent system selection that is completely aligned with QbD principles and methodology. S-Matrix's patented Trend Responses™ technology overcomes the limitations inherent in traditional approaches, and replaces a qualitative “pick-the-winner” approach with a rigorous and quantitative methodology. Most importantly, Trend Responses eliminate the requirement for laborious and error-prone peak tracking in column and solvent system screening experiments. Click here to see examples of the amazing results achieved in JUST A FEW DAYS with this approach!
Fast Chemistry System Screening
Simple Chromatogram Processing in Empower
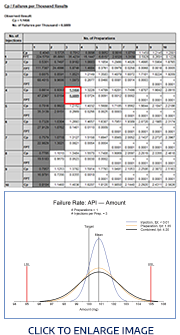
Robust Method Optimization
Fusion QbD revolutionizes method optimization with the integration of (1) flexible DOE Experimental Design, (2) automated UV and MS Based Peak Tracking, (3) retention-based Resolution Map Modeling, along with modeling all individual peak resolutions and all other required method chromatographic performance requirements (e.g. Area, Tailing, K-prime, Plates, etc.), (4) modeling all study parameter effects on all critical performance characteristics, including linear, non-linear, and Interaction Effects, and (5) the correct and regulatory accepted Integration of QbD Robustness.
Optimization with Forced Degradation Study Automation

Forced Degradation Study Automation
S-Matrix has now created a new Forced Degradation Study mode to support experiments in which the different Sample Preparation replicates represent different degradation paths such as photo degradation, acid degradation, and peroxide degradation. This activates an entirely new technology which aggregates peak data from the replicates for each run into a “Composite Chromatogram” data set for the run to be used in robust method optimization and prediction chromatogram visualization.
Automated UV and MS Based Peak Tracking
PeakTracker™ automates, optimizes, and simplifies the use of PDA and MS data in chromatographic method development. PeakTracker uses 3D PDA spectra data augmented with standard UV peak results data to automatically identify each peak in each experiment chromatogram. PeakTracker also fully utilizes 3D mass spectral data for experiments run on LC systems configured with the Waters Acquity QDa Mass Detector (QDa). Complex separation and tracking challenges PeakTracker can automatically address include:
Automated UV and MS Based Peak Tracking

- Auto-deconvolution of partially and completely co-eluted peaks.
- Two or more peaks with identical mass data.
- Non-ionizable and non-absorbing compounds.
LC Method Development for Large Molecules aligned with ICH and USP Guidances
This video presentation will illustrate using the standard method development workflow in Fusion QbD for development of Large Molecule LC methods The presentation will use a recently completed project which successfully developed a CIEX Method for mAb Charge Variant Analysis to illustrate the standard method development workflow in Fusion QbD: