S-Matrix – Software Solution Partner of:


Meets all FDA, ICH, and USP requirements and guidances, including ICH Q2(R1) and new USP <1210>.
- Small Molecule – traditional pharmaceutical substances and products
- Large Molecule – the only complete and automated method validation software for biopharmaceutical products (e.g. MAbs)
- Non-LC Methods – also used for a wide variety of non-LC methods (e.g. QNMR, CE, GC-MS, LC-MS)
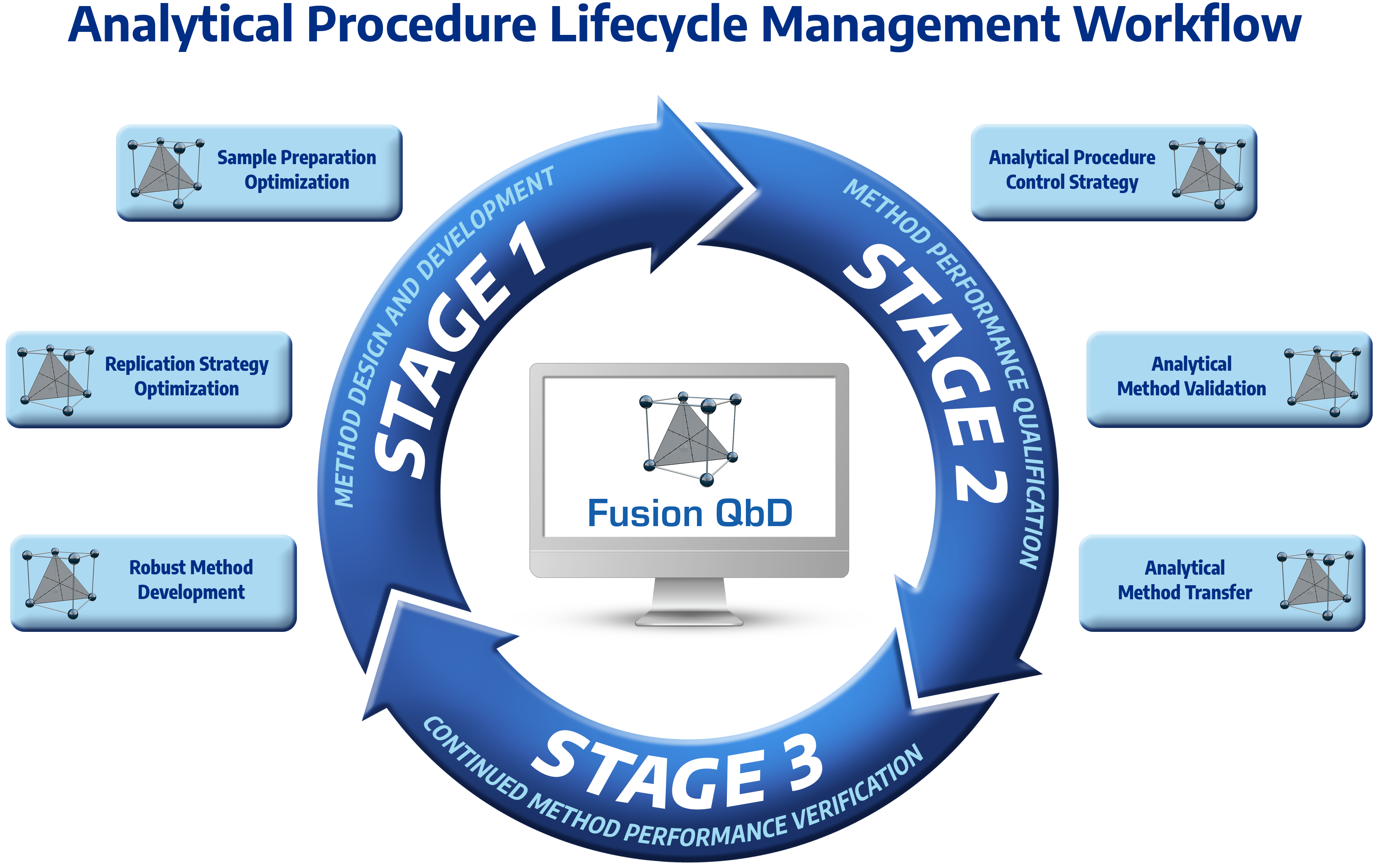
Comprehensive Method Validation Experiment Suite
- Analytical Capability*
- Specificity
- Filter Validation
- Sample Solution Stability
- Accuracy*
- Linearity and Range
- Repeatability*
- Accuracy/Linearity/Repeatability*
- [ICH-Q2(R1) – can be done as a single combined experiment]
- LOQ, LOD
- Intermediate Precision & Reproducibility (USP Ruggedness)
- Robustness – done the right way!
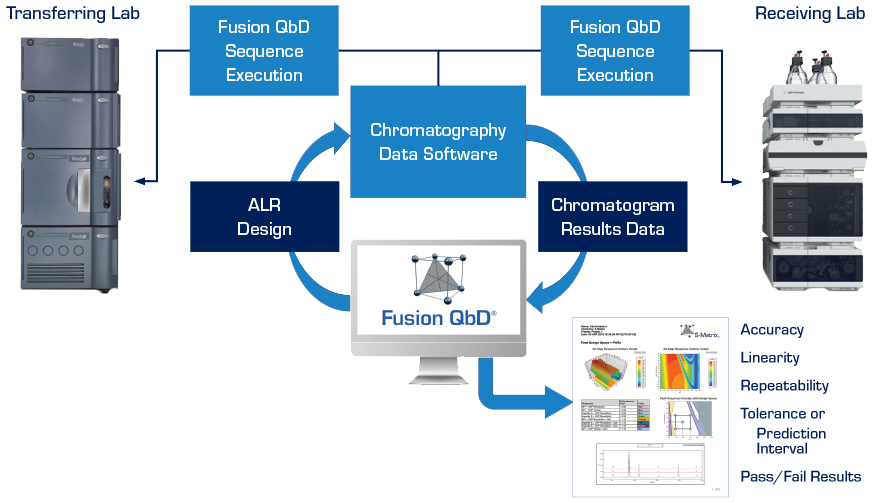
*Method Transfer Studies — Support Includes USP ⟨1210⟩
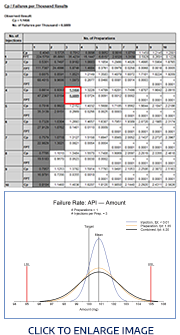
Tolerance &
Prediction Intervals
Key Benefits
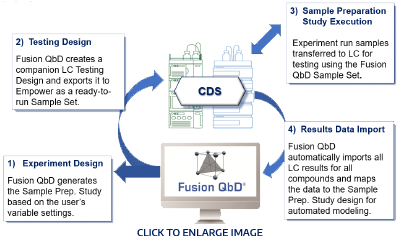
- Full Automation – Phased Method Validation
- Early Phase – performance characterization supports development
- Final Phase – Aligned with FDA and ICH guidances
- 21 CFR 11 compliance support toolset
- Including E-records and E-signatures, full audit logging
- Workflow management system with E-review and E-approve loops
- Easy setup of experiments
- Create standardized workflow templates
- Facilitate rigorous practice and defensibility
- Simple documentation review and reporting
- Easy to defend and communicate
- Reports meet all FDA and ICH guidelines